Mode of Action
 Paraquat disrupts photosynthesis in chloroplasts
Paraquat disrupts photosynthesis in chloroplasts
Paraquat acts in the presence of light to desiccate the green parts of all plants with which it comes into contact. After application, penetration through the leaf surface occurs almost immediately. This absorption is increased by high light intensity and humidity and by added non-ionic surfactant in the formulation which ensures good spray retention and wetting of target foliage.
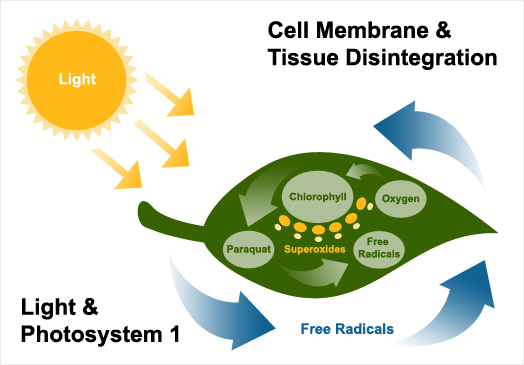
The site of action for paraquat is in the chloroplast. The chloroplasts contain the photosynthetic systems of green plants, which absorb light energy that is used to produce sugars. Paraquat is known to act on the photosynthetic membrane system called photosystem I, which produces free electrons to drive photosynthesis.
The free electrons from photosystem I react with the paraquat ion to give the free radical form. Oxygen rapidly reconverts this free radical and in that process produces super oxides. Chemically highly reactive, super oxides attack unsaturated membrane fatty acids, rapidly opening up and disintegrating the cell membranes and tissues. The paraquat ion/free radical process then recycles producing further quantities of super oxide until the supply of free electrons ceases.
Visible wilting of treated plants is apparent within hours under warm, bright conditions but may take several days under dull, cool conditions. This is quickly followed by the appearance of brown, desiccated or chlorotic tissue. Light, oxygen and chlorophyll are, therefore, all required for the rapid and characteristic herbicidal effects of paraquat.
 The chloroplast is the site of action for paraquat
The chloroplast is the site of action for paraquat
It is the rupturing of the cell membranes allowing water to escape from the plant material that leads to the rapid desiccation of the foliage. The speed of cell destruction is usually too rapid to allow any measurable translocation from the treated leaf to occur. Consequently complete coverage of the foliage and growing points is required for annual weed control. Perennial weeds can re-grow and may require further treatment.
Chemistry
Paraquat is a broad spectrum, non-selective herbicide. It belongs to the Bipyridylium family of herbicides. Pure paraquat salts are white and the technical products, yellow. They are crystalline, odorless, hygroscopic powders. Paraquat is slightly soluble in alcohol and practically insoluble in organic solvents. Paraquat is non-explosive and non-flammable in aqueous formulations. It is corrosive to metals and incompatible with alkylarylsulfonate wetting agents. It is stable in acid or neutral solutions, but is readily hydrolysed by alkali.
|
Structural formula |
Paraquat is a strong cation and stays where applied |
|
|---|---|---|
|
Description |
White crystaline solid |
like sucrose |
|
Solubility |
Very soluble in water. Not soluble in fat |
like sodium chloride |
|
Vapor pressure |
Negligible, below 1 x 10-9 mm Hg. Inhalation not possible |
like copper coins |
|
Toxicity (45.6% technical) |
Oral LD50 (technical material in the rat) = 283mg/kg |
like gasoline |
Molecular Structure
 Paraquat: 1,1'-dimethyl-4,4'-bipyridylium dichloride
Paraquat: 1,1'-dimethyl-4,4'-bipyridylium dichloride

