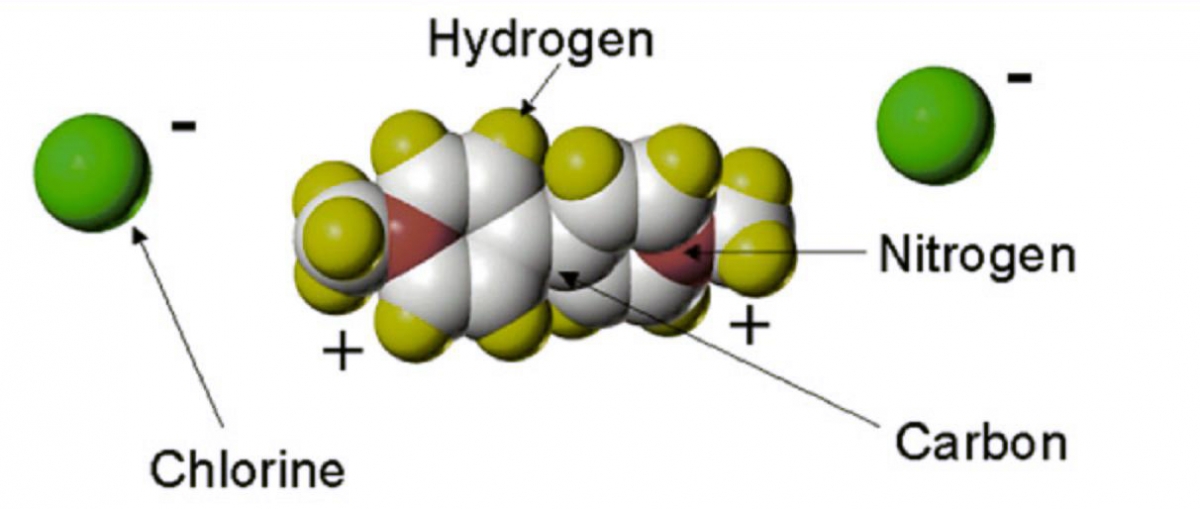
Chemical and physical properties of paraquat
The active ingredient paraquat is a non-volatile white crystalline solid, melting and decomposing at 300°C. Extremely soluble in water, it is practically insoluble in most organic solvents. Paraquat is formulated as the dichloride salt.
| Common Name: | paraquat |
|---|---|
| Chemical Name: | 1,1’-dimethyl-4,4’-bipyridylium ion |
| Structural Formula: | 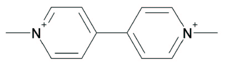 |
|
Empirical Formula: |
C12H14N2Cl2 |
| Molecular Weight: | 257 |
| Water solubility: | 620 g/l (20 ºC) |
| pH Stability: | Acidic: stable Neutral: stable Alkaline: hydrolysed |
| Vapor Pressure: | 1 x 10-9 mm Hg |
| Photostability: | Decomposed by UV radiation in aqueous solution |
Mode of Action
Paraquat acts in the chloroplasts of green plants. Here, photosynthetic systems absorb light energy to produce sugars for plant nutrition. Paraquat precisely targets the biochemical system known as Photosystem I. This produces free electrons, which drive photosynthesis. The paraquat ion reacts with these electrons to form “free radicals.” Oxygen rapidly converts free radicals to superoxides. These readily react with the unsaturated fatty acid components of cell membranes. As a result of these dramatic chemical changes, membranes are destroyed, and cell contents leak and mix causing further destruction. This entire process occurs so quickly that there is no measurable translocation of paraquat.

